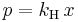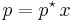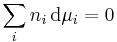Henry's law
In chemistry, Henry's law is one of the gas laws, formulated by William Henry in 1803. It states that:
- At a constant temperature, the amount of a given gas dissolved in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
An equivalent way of stating the law is that the solubility of a gas in a liquid at a particular temperature is proportional to the pressure of that gas above the liquid. Henry's law has since been shown to apply for a wide range of dilute solutions, not merely those of gases.
An everyday example of Henry's law is given by carbonated soft drinks. Before the bottle or can is opened, the gas above the drink is almost pure carbon dioxide at a pressure slightly higher than atmospheric pressure. The drink itself contains dissolved carbon dioxide. When the bottle or can is opened, some of this gas escapes, giving the characteristic hiss (or "pop" in the case of a champagne bottle). Because the pressure above the liquid is now lower, some of the dissolved carbon dioxide comes out of solution as bubbles. If a glass of the drink is left in the open, the concentration of carbon dioxide in solution will come into equilibrium with the carbon dioxide in the air, and the drink will go "flat".
Contents |
Formula and the Henry's law constant
Henry's law can be put into mathematical terms (at constant temperature) as
where p is the partial pressure of the solute in the gas above the solution, c is the concentration of the solute and kH is a constant with the dimensions of pressure divided by concentration.[1] The constant, known as the Henry's law constant, depends on the solute, the solvent and the temperature.
Some values for kH for gases dissolved in water at 298 K include:
- oxygen (O2) : 769.2 L·atm/mol
- carbon dioxide (CO2) : 29.4 L·atm/mol
- hydrogen (H2) : 1282.1 L·atm/mol
There are other forms of Henry's Law, each of which defines the constant kH differently and requires different dimensional units.[2] In particular, the "concentration" of the solute in solution may also be expressed as a mole fraction or as a molality.[1]
Other forms of Henry's law
There are various other forms Henry's Law which are discussed in the technical literature.[2][3][4]
| equation: |  |
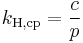 |
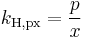 |
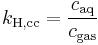 |
|---|---|---|---|---|
| units: | 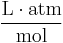 |
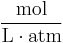 |
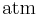 |
dimensionless |
| O2 | 769.23 | 1.3 × 10−3 | 4.259 × 104 | 3.180 × 10−2 |
| H2 | 1282.05 | 7.8 × 10−4 | 7.099 × 104 | 1.907 × 10−2 |
| CO2 | 29.41 | 3.4 × 10−2 | 0.163 × 104 | 0.8317 |
| N2 | 1639.34 | 6.1 × 10−4 | 9.077 × 104 | 1.492 × 10−2 |
| He | 2702.7 | 3.7 × 10−4 | 14.97 × 104 | 9.051 × 10−3 |
| Ne | 2222.22 | 4.5 × 10−4 | 12.30 × 104 | 1.101 × 10−2 |
| Ar | 714.28 | 1.4 × 10−3 | 3.955 × 104 | 3.425 × 10−2 |
| CO | 1052.63 | 9.5 × 10−4 | 5.828 × 104 | 2.324 × 10−2 |
where:
- c = amount concentration of gas in solution (in mol/L)
- p = partial pressure of gas above the solution (in atm)
- x = mole fraction of gas in solution (dimensionless)
As can be seen by comparing the equations in the above table, the Henry's law constant kH,pc is simply the inverse of the constant kH,cp. Since all kH may be referred to as Henry's law constants, readers of the technical literature must be quite careful to note which version of the Henry's Law equation is being used.[2]
It should also be noted the Henry's Law is a limiting law that only applies for sufficiently dilute solutions. The range of concentrations in which it applies becomes narrower the more the system diverges from ideal behavior. Roughly speaking, that is the more chemically different the solute is from the solvent.
It also only applies simply for solutions where the solvent does not react chemically with the gas being dissolved. A common example of a gas that does react with the solvent is carbon dioxide, which forms carbonic acid (H2CO3) to a certain degree with water.
Temperature dependence of the Henry constant
When the temperature of a system changes, the Henry constant will also change.[2] This is why some people prefer to name it Henry coefficient. There are multiple equations assessing the effect of temperature on the constant. These examples are derived by integrating the van 't Hoff equation:[4]
where
- kH for a given temperature is the Henry's Law constant (as defined in the first section of this article). Notice that the correct sign of C depends on whether kH,pc or kH,cp is used.
- T is the thermodynamic temperature,
- T
orefers to the standard temperature (298 K).
This equation is only an approximation, and should be used only when no better, experimentally-derived formula is known for a given gas.
The following table lists some values for constant C (in kelvins) in the equation above:
| Gas | O2 | H2 | CO2 | N2 | He | Ne | Ar | CO |
| C(K) | 1700 | 500 | 2400 | 1300 | 230 | 490 | 1300 | 1300 |
Because solubility of permanent gases usually decreases with increasing temperature at around the room temperature, the partial pressure a given gas concentration has in liquid must increase. While heating water (saturated with nitrogen) from 25 °C to 95 °C the solubility will decrease to about 43% of its initial value. This can be verified when heating water in a pot: small bubbles evolve and rise, long before the water reaches boiling temperature. Similarly, carbon dioxide from a carbonated drink escapes much faster when the drink is not cooled because of the increased partial pressure of CO2 in higher temperatures. Partial pressure of CO2 in seawater doubles with every 16 K increase in temperature.[5]
The constant C may be regarded as:
where
- ΔsolvH is the enthalpy of solution
- R is the gas constant.
The solubility of gases does not always decrease with increasing temperature. For aqueous solutions, the Henry-law constant usually goes through a maximum (i.e., the solubility goes through a minimum). For most permanent gases, the minimum is below 120 °C. It is often observed that the smaller the gas molecule (and the lower the gas solubility in water), then the lower the temperature of the maximum of the Henry-law constant. Thus, the maximum is about 30 °C for helium, 92 to 93 °C for argon, nitrogen and oxygen, and 114 °C for xenon.[6]
In geophysics
In geophysics, a version of Henry's law applies to the solubility of a noble gas in contact with silicate melt. One equation used is
where:
- C = the number concentrations of the solute gas in the melt and gas phases
- β = 1/kBT, an inverse temperature scale: kB = the Boltzmann constant
- µE = the excess chemical potentials of the solute gas in the two phases.
Comparison to Raoult's law
For a dilute solution, the concentration of the solute is approximately proportional to its mole fraction x, and Henry's law can be written as:
This can be compared with Raoult's law:
where p* is the vapor pressure of the pure component.
At first sight, Raoult's law appears to be a special case of Henry's law where kH = p*. This is true for pairs of closely related substances, such as benzene and toluene, which obey Raoult's law over the entire composition range: such mixtures are called "ideal mixtures".
The general case is that both laws are limit laws, and they apply at opposite ends of the composition range. The vapor pressure of the component in large excess, such as the solvent for a dilute solution, is proportional to its mole fraction, and the constant of proportionality is the vapor pressure of the pure substance (Raoult's law). The vapor pressure of the solute is also proportional to the solute's mole fraction, but the constant of proportionality is different and must be determined experimentally (Henry's law). In mathematical terms:
- Raoult's law:
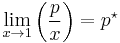
- Henry's law:
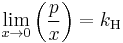
Raoult's law can also be related to non-gas solutes.
Standard chemical potential
Henry's law has been shown to apply to a wide range of solutes in the limit of "infinite dilution" (x→0), including non-volatile substances such as sucrose or even sodium chloride. In these cases, it is necessary to state the law in terms of chemical potentials. For a solute in an ideal dilute solution, the chemical potential depends on the concentration:
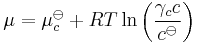 , where
, where 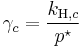 for a volatile solute; c
for a volatile solute; co= 1 mol/L.
For non-ideal solutions, the activity coefficient γc depends on the concentration and must be determined at the concentration of interest. The activity coefficient can also be obtained for non-volatile solutes, where the vapor pressure of the pure substance is negligible, by using the Gibbs–Duhem relation:
By measuring the change in vapor pressure (and hence chemical potential) of the solvent, the chemical potential of the solute can be deduced.
The standard state for a dilute solution is also defined in terms of infinite-dilution behavior. Although the standard concentration co is taken to be 1 mol/L by convention, the standard state is a hypothetical solution of 1 mol/L in which the solute has its limiting infinite-dilution properties. This has the effect that all non-ideal behavior is described by the activity coefficient: the activity coefficient at 1 mol/L is not necessarily unity (and is frequently quite different from unity).
All the relations above can also be expressed in terms of molalities rather than concentrations, e.g.:
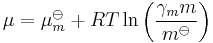 , where
, where 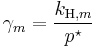 for a volatile solutes; m
for a volatile solutes; mo= 1 mol/kg.
The standard chemical potential μmo, the activity coefficient γm and the Henry's law constant kH,m all have different numerical values when molalities are used in place of concentrations.
See also
- Dalton's law
- Partial pressure
References
- ↑ 1.0 1.1 International Union of Pure and Applied Chemistry (1993). Quantities, Units and Symbols in Physical Chemistry, 2nd edition, Oxford: Blackwell Science. ISBN 0-632-03583-8. p. 50. Electronic version.
- ↑ 2.0 2.1 2.2 2.3 Francis L. Smith and Allan H. Harvey (September 2007). "Avoid Common Pitfalls When Using Henry's Law". CEP (Chemical Engineering Progress). ISSN 0360-7275.
- ↑ University of Arizona chemistry class notes
- ↑ 4.0 4.1 4.2 An extensive list of Henry's law constants, and a conversion tool
- ↑ Takahashi, T; Sutherland, SC; Sweeney, C; Poisson, A; Metzl, N; Tilbrook, B; Bates, N; Wanninkhof, R; Feely, RA; Sabine, C; Olafsson, J; Nojiri, Y "Global sea-air CO2 flux based on climatological surface ocean pCO2 and seasonal biological and temperature effects" Deep-Sea Research (Part II, Topical Studies in Oceanography) [Deep-Sea Research (II Top. Stud. Oceanogr.)] 49, 9-10, pp. 1601-1622, 2002
- ↑ P. Cohen (editor), "The ASME handbook on Water Technology for Thermal Power Systems", The American Society of Mechanical Engineers, 1989, page 442.
External links
- www.henrys-law.org - Large compilation of Henry's law constants
- Ethanol solubility in EPDM - Solubility of chemicals in polymers using Henry's law
- New 'no air tanks' diving system, based on Henry's law - An article with flash presentation.
- http://dx.doi.org/10.1021/cr60242a003 - "The solubility of gases in liquids", R. Battino and H. Lawrence Clever, Chemical Reviews, 66, 4, 395-463 (1966).
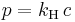
![k_{\rm H,pc}(T) = k_{\rm H,pc}(T^\ominus)\, \exp{ \left[ -C \, \left( \frac{1}{T}-\frac{1}{T^\ominus}\right)\right]}\,](/I/c7ab0d7f911ef2bd54a39a627b1c893d.png)
![k_{\rm H,cp}(T) = k_{\rm H,cp}(T^\ominus)\, \exp{ \left[ C \, \left( \frac{1}{T}-\frac{1}{T^\ominus}\right)\right]}\,](/I/53cb9ed152540ea821076c3d3f74f4ef.png)
![C = -\frac{\Delta_{\rm solv}H}{R} = -\frac{{\rm d}\left[ \ln k_{\rm H}(T)\right]}{{\rm d}(1/T)}](/I/b4568ac0d11bdf93d039d0def7bab37c.png)
![C_{\rm melt}/C_{\rm gas} = \exp\left[-\beta(\mu^{\rm E}_{\rm melt} - \mu^{\rm E}_{\rm gas})\right]\,](/I/80e500841fc90e87edd676bf0bac14d4.png)